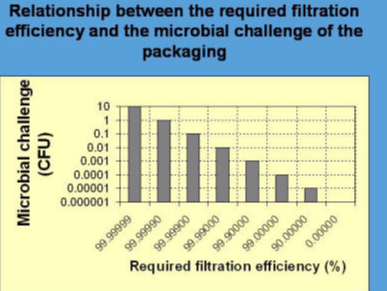
“Porous materials shall provide an adequate microbial barrier to microorganisms” – this is the statement of the International Standard ISO 11607-1 of the version 2006 and 2019 (subclause 5.2.3). It is necessary for the comparability of the barrier performances of commercially available packaging materials to use mandatorily preferred and internationally accepted standard methods. A quantitative procedure should be used, so that health care personnel can calculate the shelf-life and are able to control the maintenance of sterility at the sterility assurance level of 1:1,000,000. If manufacturers are allowed to apply significantly different test methods for the same quality parameter it would be difficult for the customer to select a commercial packaging product of the required quality level. The standard ISO 11607-1:2006 stated in regards to an adequate microbial barrier with view of the impact of sterilized packaging by airborne microorganisms:
"Evaluation of the microbial barrier properties of porous materials is typically conducted by challenging samples with an aerosol of bacterial spores or particulates under a set of test conditions …. The microbial barrier properties of the material, under these specified test conditions, are determined by comparing the extent of bacterial or particulate penetration through the material with the original challenge."
Five methods which are related to the microbial barrier and which could be used to demonstrate compliance with the requirements of ISO 11607-1 were still listed in Annex B in the 2006 version. Based on the current progress in standardization, a consensus about procedures and a harmonization of different methods is expected because comparability and scientific research are restricted when different methods have been used. Therefore, in the scope of an adequate evaluation and comparability of the barrier performances of packaging materials against airborne microorganisms, it would have been necessary from a scientific point of view that the use of airborne bacteria, spores or particles and a standard test procedure are being laid down in a binding form in future versions of ISO 11607-1 following after 2006.
However, this has not been done until now. Instead, a significant weakening to the above cited sentence can be observed in the version of 2019 compared with that of 2006:
“Evaluation of the microbial barrier properties of porous materials can be done by challenging samples with an aerosol of bacterial spores or particles, […].”
The introduction of a single and worldwide accepted standard test was also clearly called by a paper of industry representatives, entitled “Maintenance of sterility at the required sterility assurance level until the point of use - The need for suitable tests and accurate prediction” (Spencer et al. 2018):
“The absence of a single validated standard test of microbial barrier properties is an unmet need, highlighted by reported comparisons of various wrap fabric materials and whole packages based on filtration efficiencies determined by different test methods. This is confusing and unhelpful for healthcare practitioners and central sterilization workers, and discussion across the sterilization community is needed to identify by consensus a suitable test method for determining the barrier performance of the sterile packaging systems.”
Döring et al (2018) stated in this context: “Several factors can affect the sterility of cardiac implants during transport and storage. As fluctuations in temperature and air pressure lead to an inflow of airborne bacteria into the packaging, they should be minimized to the greatest extent possible. The microbe content of the ambient air, which is a variable that can be measured and controlled, also determines the degree of contamination. On the part of the manufacturers, important indicators are the air volume and filtration performance of the packaging against bacterial spores, which are measured by exposure of the packaging to bacterial spores in an aerosol. […] The manufacturer’s statements regarding these parameters are very incomplete, and the incomplete data situation prevents the microbe load from being calculated.”
In summary, tests with airborne micro-organisms or airborne particles are not prescribed explicitly as mandatory in the International Standard ISO 11607-1:2019. In consequence of this, the following critical aspects when dealing with terminally sterilized devices are adversely affected:
• Comparison and choice of commercially available sterile barrier systems by hospitals and health care facilities in regards to their barrier capacity to maintain sterility,
• choice of high effective sterile barrier systems when high risk products are used in the daily routine of patient care, e.g. cardiovascular and orthopedic implants or sterile devices that are used for dispensing sterile products in sterile containers in order to made compounded sterile preparations that are stored for extended periods before use (Staes et al, 2013),
• calculation of maintenance of sterility at the sterility assurance level on a databased procedure where the highest level of safety is required.
Various tests with different methodological procedures are listed in Annex B of the 2019 version of ISO 11607-1 under the headings of Microbial barrier and Microbial barrier surrogate. The reference methods are listed in this order (ISO 11607-1: 2019,Table B.1):
ASTM F 1608: Standard test method for microbial ranking of porous packaging materials (Exposure chamber method);
DIN 58953-6: Sterilization – sterile supply – Part 6: Microbial barrier testing of packaging materials for medical devices which are to be sterilized; subclause 3: Testing for germ proofness in moisture and subclause 4: Testing for germ proofness with passage of air;
ASTM F 2101: Test method for evaluating the bacterial filtration efficiency (BFE) of medical face masks materials, using a biological aerosol of staphylococcus aureus;
SS 876 0019: Health care textiles – bacterial penetration – Wet;
ASTM F2638: Standard Test Method for Using Aerosol Filtration for Measuring the Performance of Porous Packaging Materials as a Surrogate Microbial Barrier;
BS 6256: Specification for paper for steam sterilization paper bags, pouches and reels for medical use Annex C: Methods for determination of methylene blue particulate penetration.
One of these methods, entitled “Testing for germ proofness with passage of air” according to DIN 58953-6 is designed as pass/fail method and applies a microbiological mixture which was dissipated over the surface of the test sample and includes an air passage through the porous sample by temperature changes.
References:
Döring M, Richter S, Hindricks G. The diagnosis and treatment of pacemaker-associated infection. In reply. Dtsch Arztebl Int 2018; 115: 712
Staes C, Jacobs J, Mayer J, Allen J. Description of outbreaks of healthcare associated infections related to compounding pharmacies, 2000-2012. Am J Health Syst Pharm 2013;70:1301-1312
Spencer A St, Engels K. Maintenance of sterility at the required sterility assurance level until the point of use - The need for suitable tests and accurate prediction. Zentralsterilization 2018; 26:185-186
Dunkelberg H. Sterile supply of medical devices and pharmaceutical products. Quality standards and applied risk management. Pharm Ind 2016;78:1644-484
Dunkelberg H. Monitoring Sterile Pacemaker Implants. Dtsch Arztebl Int. 2018;115(42):712
Dunkelberg H. Long-term monitoring of airborne microbial challenge and use of cold atmospheric air plasma technology - basis for a data-based sterile starage management according to sthe sterility assurance level of 1:1,000,000. Pharm Ind 2019;81:1130-36