Attainment of the microbiological quality during transport and storage of sterile medical devices
Even packaging material with a high barrier performance against airborne micro-organisms can lead to the loss of sterility at the level of 1:1,000,000 for a storage period of months or years if the ambient airborne microbial concentration is in the range of several hundreds of CFU/m³.
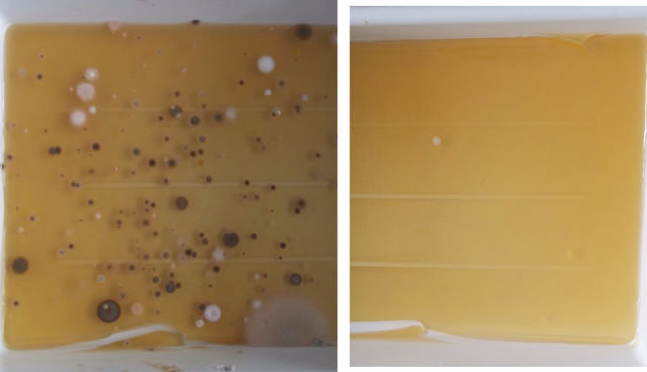
One problem-solving approach to ensure sterile quality could be obtained by reduction and control of the environmental airborne microbial concentration.
Cold atmospheric air plasma sterilization allows inactivation of airborne microorganisms such as vegetative bacterial species and spores. It operates at room temperature and inactivates micro-organisms within a short contact time. We tested this method in order to reduce continuously the airborne microbial load in a storage cabinet placed in our laboratory. The low temperature plasma sterilizer operated with an airflow of about 200 m³/h. The settle plate method was used to control the microbiological cleanliness within the cabinet. Both two dishes of 15 x 20 cm were filled with 300 ml of CASO-agar and sterilized in rigid sterilization container. Each two dishes were uncovered and exposed for about 4 days inside the cabinet and outside as control. Exposed plates were replaced by fresh ones in four stages so that the total exposure time of 2 weeks was reached (Dunkelberg, 2019).